From University of Michigan 08/04/24

A new “atlas” of the human ovary provides insights that could lead to treatments restoring ovarian hormone production and the ability to have biologically related children, according to University of Michigan engineers.
This deeper understanding of the ovary means researchers could potentially create artificial ovaries in the lab using tissues that were stored and frozen before exposure to toxic medical treatments such as chemotherapy and radiation.
Currently, surgeons can implant previously frozen ovarian tissue to temporarily restore hormone and egg production.
However, this does not work for long because so few follicles—the structures that produce hormones and carry eggs—survive through reimplantation, the researchers say.
The new atlas reveals the factors that enable a follicle to mature, as most follicles wither away without releasing hormones or an egg.
Using new tools that can identify what genes are being expressed at a single-cell level within a tissue, the team was able to home in on ovarian follicles that carry the immature precursors of eggs, known as oocytes.
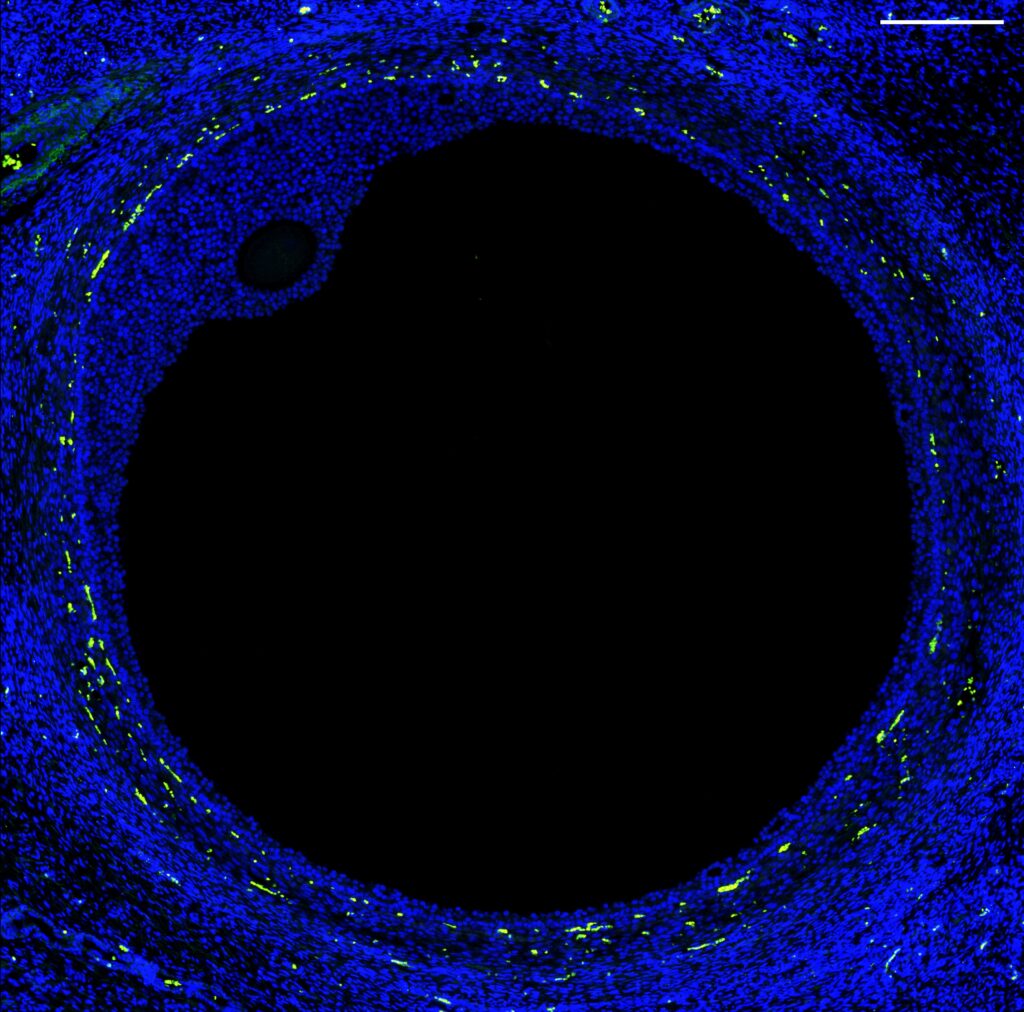
“Now that we know which genes are expressed in the oocytes, we can test whether affecting these genes could result in creating a functional follicle.”
“This can be used to create an artificial ovary that could eventually be transplanted back into the body,” said Ariella Shikanov, U-M associate professor of biomedical engineering and corresponding author of the new study in Science Advances.
The majority of the follicles, called primordial follicles, remain dormant and are located in the outer layer of the ovary, called the cortex.
A small portion of these follicles activate periodically and migrate into the ovary, to a region known as the growing pool.
Only a few of those growing follicles go on to produce mature eggs that get released into the fallopian tube.
With the ability to guide follicle development and tune ovarian environment, the team believes that engineered ovarian tissue could function for much longer than unmodified implanted tissue.
This means that patients would have a longer fertility window as well as a longer period in which their bodies produce hormones that help regulate the menstrual cycle and support muscular, skeletal, sexual and cardiovascular health.
“We’re not talking about utilizing a surrogate mother, or artificial insemination,” said Jun Z. Li, associate chair of U-M’s Department of Computational Medicine and Bioinformatics and co-corresponding author of the study.
“The magic we’re working toward is being able to trigger an immature cell into maturity, but without knowing which molecules drive that process, we’re blind.”
U-M’s team utilized a relatively new technology, called spatial transcriptomics, to track all of the gene activity—and where it occurs—in tissue samples.
They do this by reading strands of RNA, which are like notes taken from the DNA strand, revealing which genes are being read.

Working with an organ procurement organization, U-M researchers performed RNA sequencing of ovaries from five human donors.
“This was the first time where we could target ovarian follicles and oocytes and perform a transcription analysis, which enables us to see which genes are active,” Shikanov said.
“The majority of ovarian follicles, already present at birth, never enter the growing pool and eventually self-destruct.”
“This new data allows us to start building our understanding of what makes a good egg—what determines which follicle is going to grow, ovulate, be fertilized and become a baby.”
U-M’s work is part of the Human Cell Atlas project, which seeks to create “maps of all the different cells, their molecular characteristics and where they are located, to understand how the human body works and what goes wrong in disease.”
Shikanov, Li and U-M collaborators such as Sue Hammoud, U-M associate professor of human genetics and urology, are mapping other parts of the female reproductive system, including the uterus, fallopian tubes and ovaries.
Other contributors include Andrea Suzanne Kuliahsa Jones, formerly of U-M and now at Duke University, and D. Ford Hannum, a U-M graduate student research assistant in bioinformatics.
The research was partially funded by the Chan Zuckerberg Initiative.
Additional financial support was provided by the National Institutes of Health.