From American Chemical Society 19/03/24
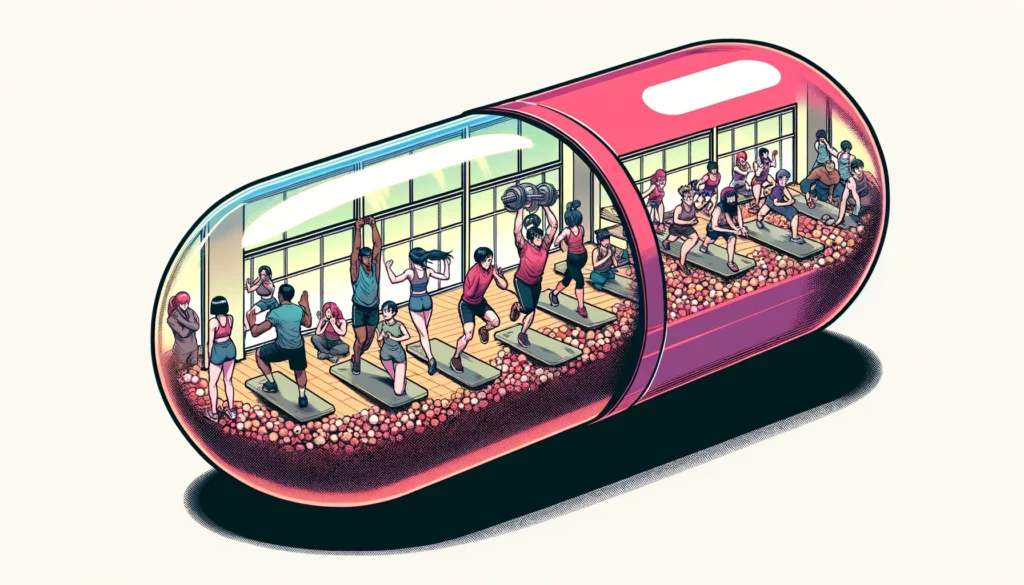
NEW ORLEANS, March 18, 2024 — Doctors have long prescribed exercise to improve and protect health.
In the future, a pill may offer some of the same benefits as exercise.
Now, researchers report on new compounds that appear capable of mimicking the physical boost of working out — at least within rodent cells.
This discovery could lead to a new way to treat muscle atrophy and other medical conditions in people, including heart failure and neurodegenerative disease.
The researchers will present their results today at the spring meeting of the American Chemical Society (ACS).
ACS Spring 2024 is a hybrid meeting being held virtually and in person March 17-21; it features nearly 12,000 presentations on a range of science topics.
“We cannot replace exercise; exercise is important on all levels,” says Bahaa Elgendy, the project’s principal investigator who is presenting the work at the meeting.
“If I can exercise, I should go ahead and get the physical activity. But there are so many cases in which a substitute is needed.”
Exercise benefits both mind and body.
In this case, Elgendy, a professor of anesthesiology at Washington University School of Medicine in St. Louis, and his colleagues are hoping to recapitulate its potent physical effects — namely, exercise’s ability to enhance muscle cells’ metabolism and growth, along with improved muscle performance.
A drug that can mimic these effects could offset the muscle atrophy and weakness that can occur as people age or are affected by cancer, certain genetic conditions or other reasons they are unable to carry out regular physical activity.

It could also potentially counter the effects of other drugs, such as new weight-loss medications that cause the loss of both fat and muscle, according to Elgendy.
The metabolic changes associated with exercise kick off with the activation of specialized proteins, known as estrogen-related receptors (ERRs), which come in three forms: ERRα, ERRβ and ERRγ.
After about a decade of work, Elgendy and his colleagues developed a compound named SLU-PP-332, which activates all three forms, including the most challenging target, ERRα.
This type of ERR regulates exercise-induced stress adaptation and other important physiological processes in muscle.
In experiments with mice, the team found this compound increased a fatigue-resistant type of muscle fiber while also improving the animals’ endurance when they ran on a rodent treadmill.
To identify SLU-PP-332, the researchers scrutinized the structure of the ERRs and how they bind to molecules that activate them.
Then, to improve upon their discovery and develop variations that could be patented, Elgendy and his team designed new molecules to strengthen the interaction with the receptors and thus provoke a stronger response than what SLU-PP-332 can provide.
When developing the new compounds, the team also optimized the molecules for other desirable characteristics, such as stability and low potential for toxicity.
The team compared the potency of SLU-PP-332 with that of the new compounds by looking at RNA, a measure of gene expression, from about 15,000 genes in cells from rat heart muscle.
The new compounds prompted a greater increase in the presence of the RNA, suggesting they more potently simulate the effects of exercise.
Research using SLU-PP-332 suggests targeting ERRs could be useful against specific diseases.
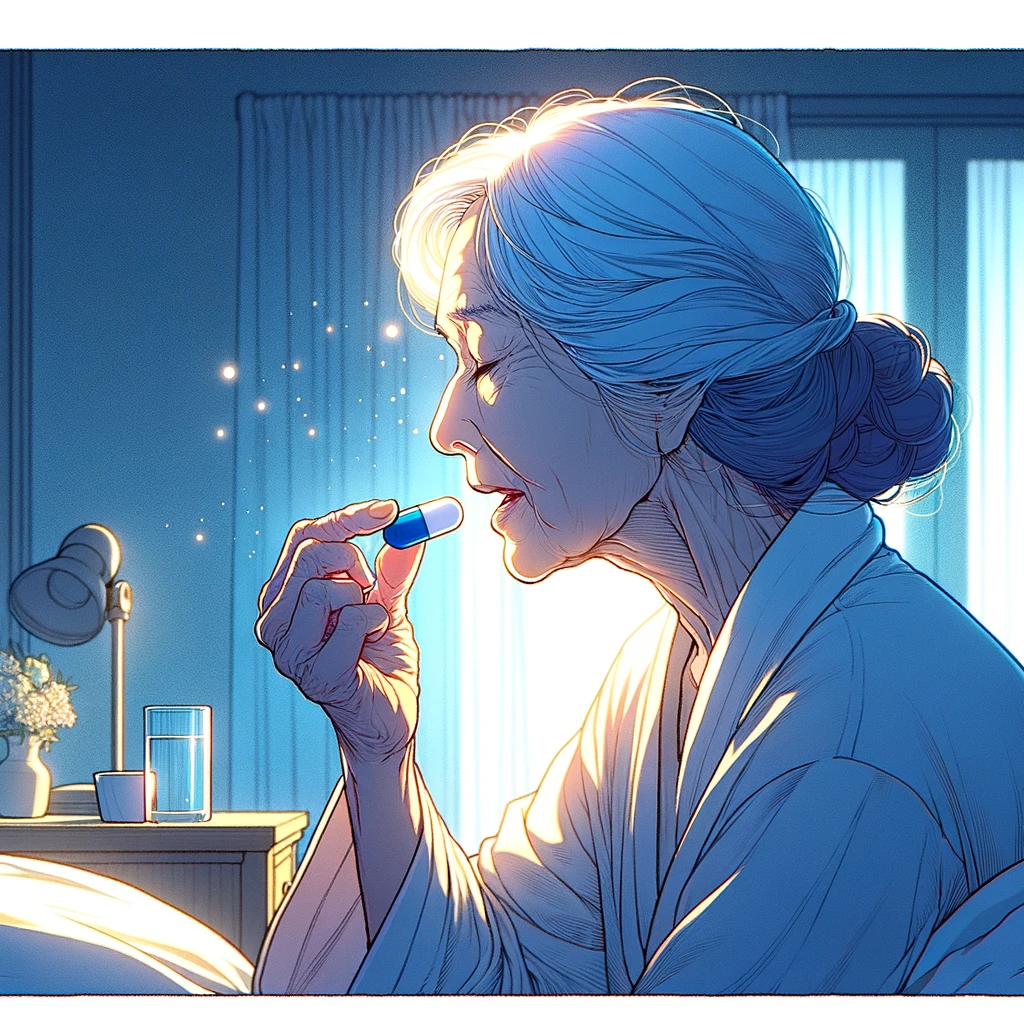
Studies in animals with this preliminary compound indicate that it could have a benefit against obesity, heart failure or a decline in kidney function with age.
The results in the updated research suggest the new compounds could have similar effects.
ERR activity also appears to counter damaging processes that occur in the brain in patients diagnosed with Alzheimer’s disease and those who have other neurodegenerative conditions.
While SLU-PP-332 cannot pass into the brain, some of the new compounds were developed to do so.
“In all of these conditions, ERRs play a major role,” Elgendy says.
“If you have a compound that can activate them effectively, you could generate so many beneficial effects.”
Elgendy and his colleagues hope to test the new compounds in animal models through Pelagos Pharmaceuticals, a startup company they have co-founded.
They are also looking into the possibility of developing the compounds as potential treatments for neurodegenerative disorders.
The research was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers R21AG065657 and RF1AG077160.