From DOE/SLAC National Accelerator Laboratory 15/09/23

Billions of tiny particles packed into rechargeable lithium-ion battery electrodes are responsible for storing charge and making it available when it’s needed to do work.
X-ray movies of this process show the particles absorbing and releasing lithium ions as the battery charges and discharges.
Now, in an important step forward, researchers have used a type of machine learning called “computer vision” to dig even deeper, analyzing each and every pixel of those X-ray movies to discover physical and chemical details of battery cycling that couldn’t be seen before.
The new method has already suggested a way to make the billions of nanoparticles in one type of lithium-ion battery electrode store and release charge more efficiently, researchers from the Department of Energy’s SLAC National Accelerator Laboratory, Stanford University, the Massachusetts Institute of Technology and Toyota Research Institute reported in Nature today.
“Until now, we could make these beautiful X-ray movies of battery nanoparticles at work, but the movies were so information-rich that understanding the subtle details of how the particles function was a real challenge,” said William Chueh, a Stanford associate professor, SLAC faculty scientist and director of the SLAC-Stanford Battery Center, who co-led the study with MIT Professor Martin Bazant.
“Now we can extract insights that were not possible before,” Chueh said.
“This is the kind of fundamental, science-based information that our partners in industry need to develop better batteries faster.”
More broadly, the researchers said, this approach to discovering the physics behind complex patterns in images could even provide unprecedented insights into other types of chemical and biological systems, such as cells dividing in a developing embryo.
See-through batteries give up their secrets
The battery particles the research team studied are made of lithium iron phosphate, or LFP.
They’re packed by the billions into the positive electrodes of many lithium-ion batteries, each one coated with a thin layer of carbon to improve the electrode’s electrical conductivity.
To watch what’s happening inside the battery while it operates, Chueh’s team builds tiny, transparent cell batteries in which two electrodes are surrounded by an electrolyte solution full of free-moving lithium ions.
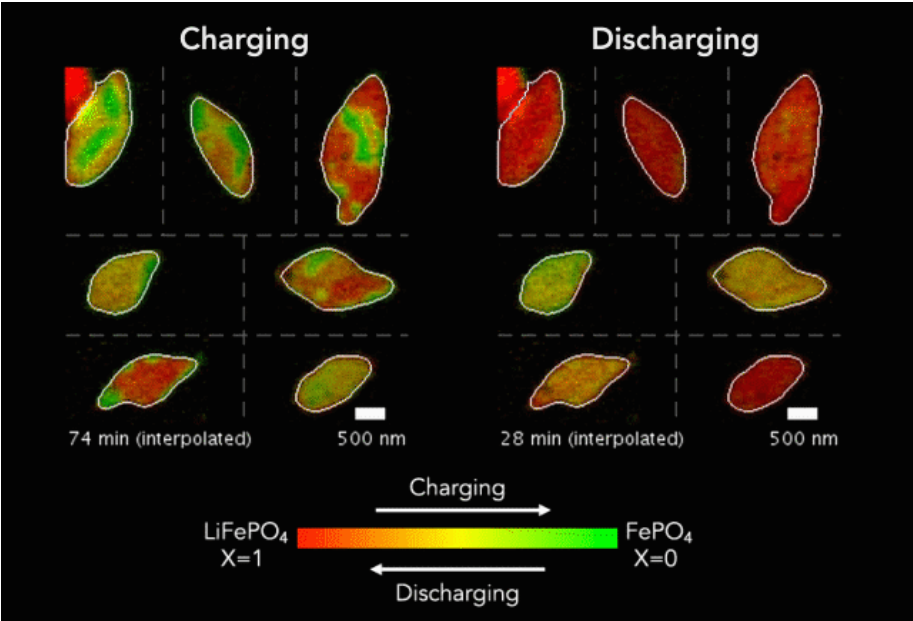
When the battery discharges, lithium ions flow into the positive LFP electrode and lodge inside its nanoparticles like cars in a crowded parking garage, in a reaction called intercalation.
When the battery charges, they flow back out again and travel to the opposite, negative electrode.
“Lithium iron phosphate is an important battery material due to low cost, a good safety record and its use of abundant elements,” said Brian Storey, senior director of Energy and Materials at the Toyota Research Institute, which funded the work at SLAC and MIT.
“We are seeing an increased use of LFP in the electric vehicle market, so the timing of this study could not be better.”
Chueh and Bazant started collaborating on battery research eight years ago.
Bazant had already done a lot of mathematical modeling of patterns formed by lithium ions as they move in and out of LFP particles.
Chueh had been using an advanced X-ray microscope at Lawrence Berkeley National Laboratory’s Advanced Light Source to make nanoscale movies, with details as small as billionths of a meter, of battery particles at work.
In 2016, their research teams published groundbreaking nanoscale movies of how lithium ions flow in and out of individual LFP nanoparticles.
Then, with funding from Toyota Research Institute, the team started using machine learning tools developed at MIT to dramatically accelerate both battery testing and the process of winnowing down many possible charging methods to find the ones that work best.
They also combined conventional machine learning, which looks for patterns in data, with knowledge gained from experiments and equations guided by physics to discover and explain a process that shortens the lifetimes of fast-charging lithium-ion batteries.
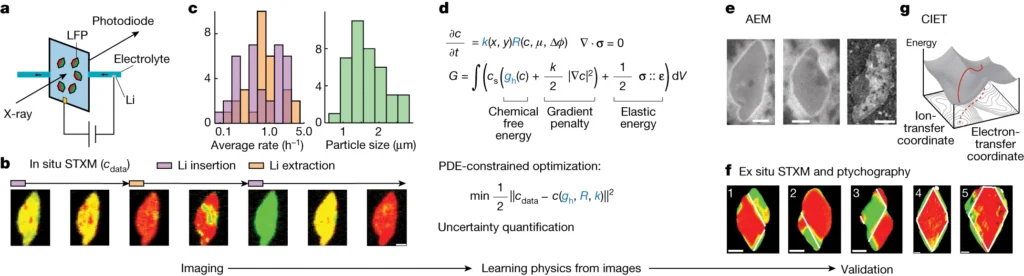
Workflow of learning the physics of battery nanoparticles from images. Credit: Nature
A pixel-by-pixel analysis
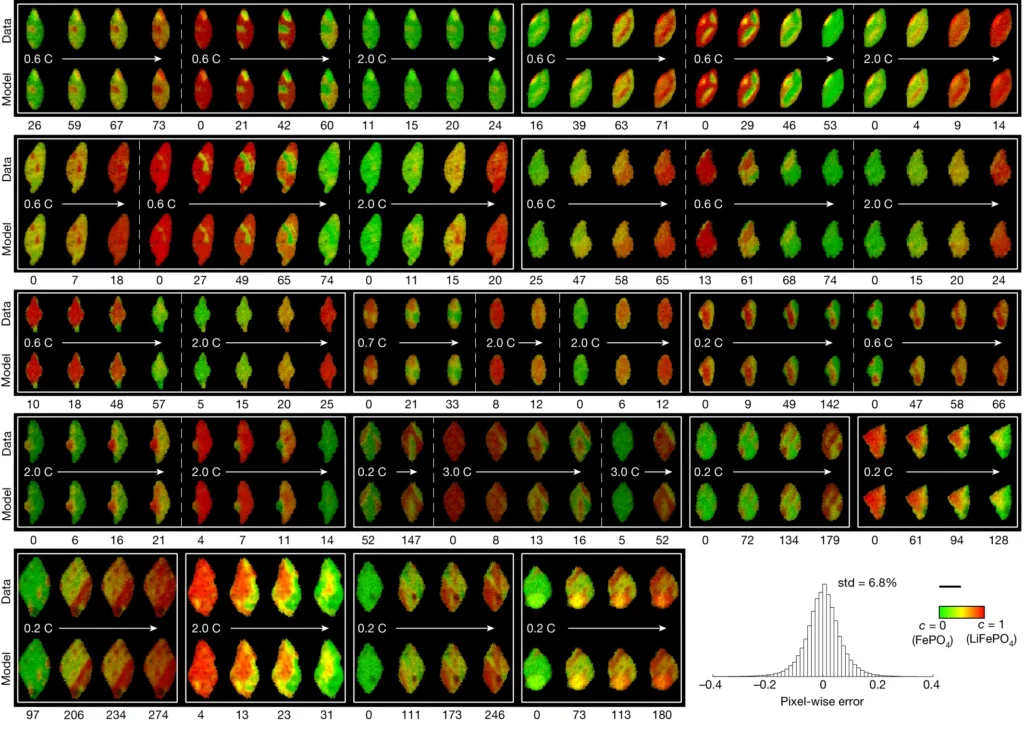
In this latest study, Chueh and Bazant used a subfield of machine learning called computer vision to mine more detailed information from 62 of the nanoscale X-ray movies they made in 2016 of LFP particles charging or discharging.
Each still image from those movies contained roughly 490 pixels – the smallest units of information that can be obtained from an image, whether it’s made with X-ray light hitting a detector or with visible light hitting a smartphone camera. This gave them about 180,000 pixels of information to work with.
The team used those 180,000 pixels to train their computational model to produce equations that accurately described how the lithium insertion reactions proceed.
They discovered that the ions’ movements within the LFP particles closely matched the predictions of Bazant’s computer simulations.
“Every little pixel in there is jumping from full to empty, full to empty,” Bazant said. “And we’re mapping that whole process, using our equations to understand how that’s happening.”
The new technique has revealed several phenomena that couldn’t be seen before, including variations in the rate of lithium insertion reactions in different regions of a single LFP nanoparticle.
“There are regions that seem to be fast,” Bazant said, “and others that seem to be slow.”
The paper’s most significant practical finding – that variations in the thickness of an LFP particle’s carbon coating directly control the rate at which lithium ions flow in and out – could lead to more efficient charging and discharging.
What the scientists learned from this study, Bazant said, is that it’s the interface between the liquid electrolyte and the solid electrode materials – where the intercalation reaction and variations in the thickness of the particles’ carbon coating interact in complicated ways – that controls battery processes. “That means that our focus should really be on engineering that interface,” he said.
Toyota Research Institute’s Storey added, “This publication is the culmination of six years of dedication and collaboration.
This technique allows us to unlock the inner workings of the battery in a way not previously possible. Our next goal is to improve battery design by applying this new understanding.”