Scientists at The Hospital for Sick Children (SickKids) in Canada have uncovered the atomic structure of a neuron communication enzyme, advancing understanding of synaptic function for neurological conditions.
From The Hospital for Sick Children 26/06/24
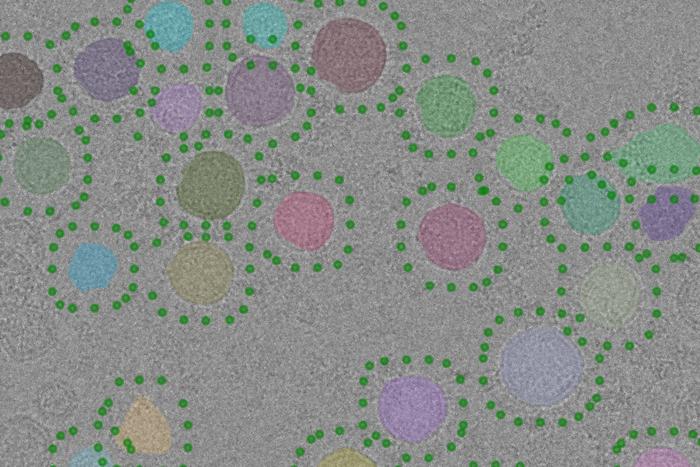
For the first time, scientists at The Hospital for Sick Children (SickKids) used advanced imaging technology at the SickKids Nanoscale Biomedical Imaging Facility to reveal the atomic structure of an enzyme that neurons use to communicate.
All brain activity – from memory and emotion to learning and motor control – is made possible through communication across synapses, the connections between neurons.
When this communication is unsuccessful, various conditions such as epilepsy can occur.
A neuron is a type of cell that specializes in communicating with other cells by sending out chemical signals, called neurotransmitters, into synapses.
In the brain, there are 100 trillion synapses between neurons.
The way neurons communicate has been studied for decades, but research published today in Science showcases models derived from hundreds of thousands of high-resolution images that reveal synaptic function with new clarity.
Led by Dr. John Rubinstein, Senior Scientist in the Molecular Medicine program, and Dr. Claire Coupland, first author and postdoctoral fellow in the Rubinstein Lab, the research team hopes that by capturing images of and modeling how chemicals are released from neurons, they may be able to inform new therapeutic targets that help improve care for children with epilepsy and other neurological conditions.
On the publication of these findings, Rubinstein shares how his team captured the images, and what their findings could mean for patients in the future.
What did your research uncover about the way neurons communicate?
When communicating, neurons release neurotransmitters into a synapse to be delivered to a receiving neuron.
These neurotransmitters are released from small packets called synaptic vesicles.
Once a message is received, the neurotransmitters must be reabsorbed and repackaged into new synaptic vesicles to clear out the synapse and make room for the next signal.
To facilitate this process, an enzyme called the vesicular-type ATPase (V-ATPase) acts as a pump to drive neurotransmitters into synaptic vesicles.
V-ATPase also regulates neurotransmitter release from the vesicles.
In our research, we learned that the way V-ATPase controls the process of neurotransmitter release from synaptic vesicles is by spontaneously falling apart after the vesicles are loaded.
We found that when we filled the synaptic vesicles with neurotransmitters, the V-ATPases split into two parts, which then allows neurotransmitter release.
How did you capture images of this process?
By using novel biochemical methods and novel imaging methods supported by the SickKids Nanoscale Biomedical Imaging Facility, we were able to isolate synaptic vesicles and obtain images of them.
From there, we developed new computational approaches to analyze the images to show the V-ATPase in the vesicles at high resolution – something that has not been done before.
We created 3D models of the V-ATPase based on images we captured using cryogenic electron microscopy (cryo-EM), a method that images samples at –196 C.
Our team saw that V-ATPase interacts with several components of the synaptic vesicle, which contains many proteins and lipids that are involved in neurotransmitter release.
Most surprisingly, we learned that the V-ATPase interacts with a protein called synaptophysin.
By weight, synaptophysin is the most abundant synaptic vesicle protein.
Until now, its function in neurons was not understood. What we found shows that synaptophysin could be helping to recruit V-ATPase to synaptic vesicles when they initially form.
What are the next steps for this research?
Now that we have discovered that V-ATPase interacts with synaptophysin in synaptic vesicles, we are working with Dr. Lu-Yang Wang, a Senior Scientist in the Neurosciences & Mental Health program, to understand the role of this interaction in the brain.
We also want to understand how the loading of vesicles leads to the V-ATPase falling apart, and how this process controls the release of neurotransmitters from neurons.
In the future, this process could be a therapeutic target for many health conditions, including some kinds of epilepsy.
This research was funded by the Canadian Institutes of Health Research (CIHR), University of Toronto and the Natural Sciences and Engineering Research Council (NSERC). Infrastructure in the Nanoscale Biomedical Imaging Facility was supported by the Canada Foundation for Innovation and the Ontario Research Fund.