From Cell Press 11/09/23
Guangzhou Institutes of Biomedicine and Health researchers have successfully created chimeric embryos containing a combination of human and pig cells.
When transferred into surrogate pig mothers, the developing humanized kidneys had normal structure and tubule formation after 28 days.
This is the first time that scientists have been able to grow a solid humanized organ inside another species, though previous studies have used similar methods to generate human tissues such as blood or skeletal muscle in pigs.
The researchers focused on kidneys because they are one of the first organs to develop, and they’re also the most commonly transplanted organ in human medicine.
“Rat organs have been produced in mice, and mouse organs have been produced in rats, but previous attempts to grow human organs in pigs have not succeeded,” says senior author Liangxue of the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, and Wuyi University.
“Our approach improves the integration of human cells into recipient tissues and allows us to grow human organs in pigs.”
Integrating human stem cells into pig embryos has been a challenge because pig cells outcompete human cells and pig and human cells have different physiological needs.
“We have been working on mechanisms to overcome the extremely low efficiency in interspecies chimera,” says senior author Guangjin Pan of the Guangzhou Institutes of Biomedicine and Health.
“We identified a couple of critical factors that enhance the formation of interspecies chimera by facilitating cell competition.”
The team’s technique depends on three key components:
First, they created a niche within the pig embryo so that the human cells would not have to compete with pig cells by using CRISPR to genetically engineer a single-cell pig embryo so that it was missing two genes that are needed for kidney development.
Second, the researchers engineered human pluripotent stem cells—cells that have the potential to develop into any cell type—to make them more amenable to integration and less likely to self-destruct by temporarily shutting down apoptosis.
Then, they converted these cells into “naïve” cells resembling early human embryonic cells by culturing them in a special medium.
Third, before implanting the developing embryos in surrogate sows, the researchers grew the chimeras in conditions that were optimized to provide unique nutrients and signals to both the human and pig cells, since these cells usually have disparate needs.
Altogether, the researchers transferred 1,820 embryos to 13 surrogate mothers.
After either 25 or 28 days, they terminated gestation and extracted the embryos to assess whether the chimeras had successfully produced humanized kidneys.
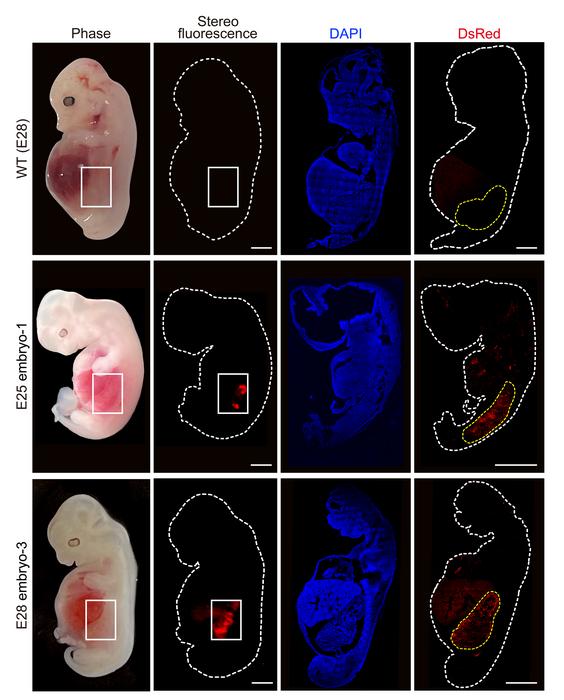
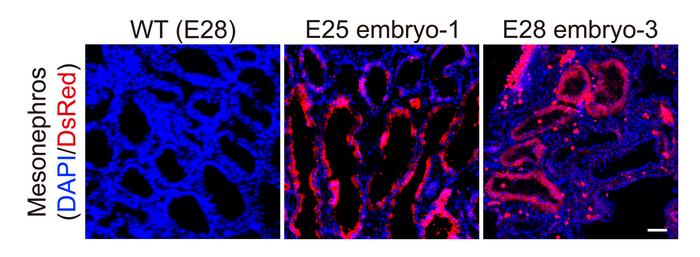
The researchers collected five chimeric embryos for analysis (two at 25 days and three at 28 days post-implantation) and found that they had structurally normal kidneys for their stage of development and were composed of 50-60% human cells.
At 25–28 days, the kidneys were in the mesonephros stage (the second stage of kidney development); they had formed tubules and buds of cells that would eventually become ureters connecting the kidney to the bladder.
The team also investigated whether human cells were contributing to other tissues throughout the embryos, which could have ethical implications, especially if abundant human cells were found in neural or germline tissues and the pigs were brought to term.
They showed that human cells were mostly localized to the kidneys, whereas the remainder of the embryo was composed of pig cells.
“We found that if you create a niche in the pig embryo, then the human cells naturally go into these spaces,” says senior author Zhen Dai of Guangzhou Institutes of Biomedicine and Health.
“We saw only very few human neural cells in the brain and spinal cord and no human cells in the genital ridge, indicating that the human pluripotent stem cells did not differentiate into germ cells.”
This may be further prevented by knocking out further genes in the human pluripotent stem cells, which can be tested in future studies, the researchers say.
Now that they have optimized conditions for growing humanized kidneys in human-pig chimeras, the team wants to allow the kidneys to develop for a longer duration.
They’re also working to generate other human organs in pigs, including the heart and pancreas.
The long-term goal is to optimize this technology for human organ transplantation, but the researchers acknowledge the work will be complex and could take many years.
Growing a fully functional humanized organ in a pig would require some additional steps because organs are composed of multiple types of cells and tissues.
In this study, the researchers created a niche for only one subset of cells, which meant that the kidneys had pig-derived vascular cells, and this could cause organ rejection if they were used in a transplant scenario.
“Because organs are not composed of just one cell lineage, in order to have an organ where everything comes from the human, we would probably need to engineer the pigs in a much more complex way and that also brings some additional challenges,” says senior author Miguel A. Esteban of Guangzhou Institutes of Biomedicine and Health.
In the meantime, this technology could be used to study the development of human organs and developmental diseases.
“Before we get to that late state of making organs that can be on the shelf for clinical practice, this method provides a window for studying human development,” says Esteban.
“You can trace the human cells you’re injecting and manipulate them so that you can study diseases and how cell lineages are formed.”