From University of Illinois Grainger College of Engineering 12/01/24
The world is filled with a myriad of sounds and vibrations.
The gentle tones of a piano drift down the hall, the relaxing purr of a cat lays on your chest, the annoying hum of office lights is present.
Imagine being able to selectively tune out noises of a certain frequency.
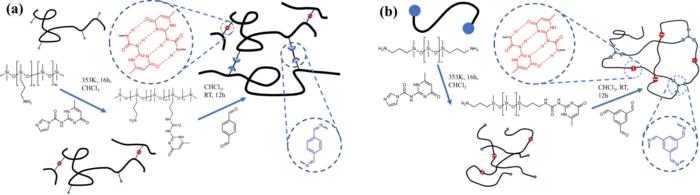
Researchers at the University of Illinois Urbana-Champaign have synthesized polymer networks with two distinct architectures.
These architectures and crosslink points are capable of dynamically exchanging polymer strands.
This research helps understand how network connectivity and bond exchange mechanisms govern the overall damping behavior of the network.
The incorporation of dynamic bonds into the polymer network demonstrates excellent damping of sound and vibrations at well-defined frequencies.
“This research is about using polymers to absorb various sounds and vibrations that can occur at different frequencies,” says materials science and engineering professor Chris Evans, who led this work.
“We want to know how to design the molecular-scale chemistry of the polymer in such a way that we control what sort of energy absorbing ability it has.”
The results of this new research were recently published in Nature Communications.
Being able to tailor polymers to absorb specific frequencies can be beneficial.
This is useful for ear plugs and helmets for people near blasts or explosions.
It is also beneficial in scenarios with repeat exposure to a certain frequency of noise, like a helicopter pilot.
Such long-term exposure can lead to hearing problems.
Polymers are long chain molecules composed of many repeating units.
Some polymers are not fully linear and have branches, like trees.
Other polymers are highly cross-linked where individual polymer chains are connected by covalent bonds to other chains, like a net.
The cross-link point is a bond that links one polymer chain to another, and this is where bonds can exchange.
Dynamic bonds within a polymer network allow it to rearrange its structure in response to a change in environment (high temperature, pH, UV light exposure, etc.).
Replacing a few covalent bonds in cross-linked polymer structures with dynamic bonds can enhance the properties of the polymer.
These properties include the modulus—the stiffness of the material—and the viscosity—how easily the material flows.
Dynamic bonds provide materials with unique properties such as self-healing, super-stretchability, adhesive properties, and material toughness due to the modification of the viscoelastic properties.
“The key advance here is that we’re using dynamic covalent bonds,” Evans explains.
“They’re chemical bonds but they can exchange with each other (the dynamic part) and when two different chemistries are used; they can exchange at very different timescales (the orthogonal part).”
“We’re using that process to try and control what frequencies of sound and vibration we’re absorbing.”
Incorporating orthogonal bonds, where fast bonds can only exchange with other fast bonds and slow bonds can only exchange with other slow bonds, generates multiple and well-separated relaxation modes.
This gives the network excellent damping and improved mechanical properties, like toughness.
The team made a series of polymers that had controlled types of architectures and backbones.
They looked at the way that the polymer chains are connected.
Evans says that it actually makes a big difference how the polymer chains are connected in order to get the energy dissipating processes at very specific timescales.
This corresponds to very specific sound waves or vibrations.
If the chains are only linked at the ends, this is not as effective as being linked periodically along the chain backbone.
One of the main limitations with the materials used in this research, however, is that they do ultimately flow.
For example, rubber bands will retain their shape, but when those dynamic bonds are added in, they’ll always eventually flow, like silly putty.
This is fine for, say, a soldier’s helmet where the material is enclosed within the shell of the helmet, but not so much for an ear plug.
Evans says his group is working on ways to get the polymer to be more of a self-standing material.
In the future, they’d like to incorporate more dynamic bonds.
So the polymer isn’t just tailored for a specific frequency, but for a much wider range of frequencies.



