From University of Oxford 07/12/23
A new study led by the University of Oxford has shed light on why certain species of bacteria carry astonishing arsenals of weapons.
The findings, published today in the journal Nature Ecology & Evolution, could help us to engineer microbes that can destroy deadly pathogens, reducing our reliance on antibiotics.
Many species of bacteria possess multiple weapons to attack competitors.
These include both short-range weapons that require direct contact with neighbouring cells, and long-range weapons, such as toxins that are released into the environment.
Up to now, why bacteria have evolved to carry such a wide array of weapons has been a mystery.
Study co-author Professor Kevin Foster (Departments of Biology and Biochemistry, University of Oxford), said: ‘Unlike animals, which tend to carry a single weapon type such as horns, antlers, or tusks, bacterial species commonly carry multiple weapons.
But it was unclear what the evolutionary basis for this was – why not just invest in a single type?
One theory was that bacteria carry multiple weapons because they serve different functions during competition.’
The researchers tested this using the opportunistic pathogen Pseudomonas aeruginosa, a priority one pathogen by the World Health Organization, due to the rapid emergence of multidrug-resistant strains.
P. aeruginosa possesses diverse weapons, including the ability to produce various toxic molecules (a long-range weapon), and toxin-loaded filaments anchored to its outer membrane (a short-range weapon).
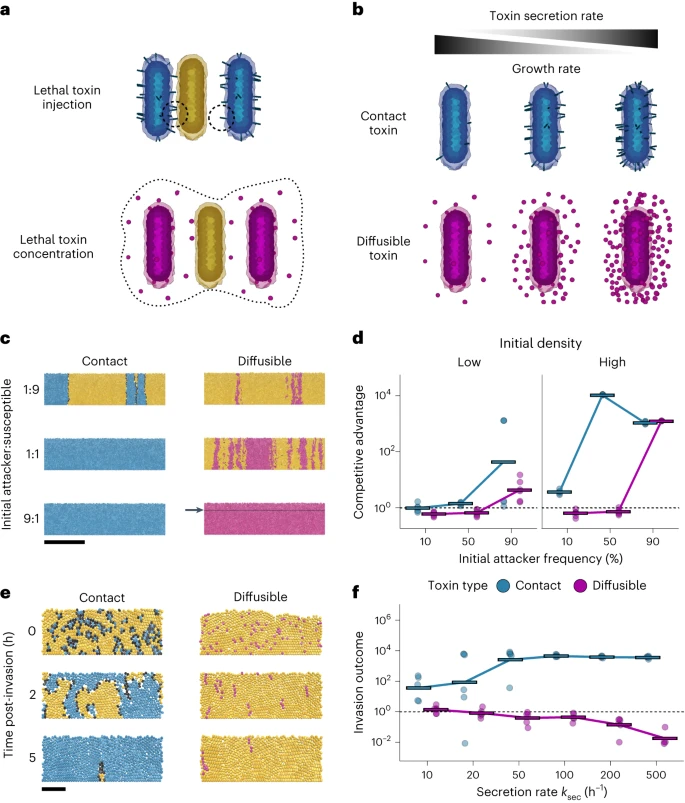
The team designed a series of experiments to determine under which conditions short- versus long-range weapons give a greater advantage.
They used genome editing to generate P. aeruginosa strains that lacked and were susceptible to either the toxin-loaded filaments or long-range toxins called tailocins.
The susceptible strains were then grown on agar plates with control P. aeruginosa over two days, at a series of different ratios.
Because the strains each expressed a different fluorescent protein, the researchers could quantify the ratio of attacker vs susceptible bacteria.
The results clearly demonstrated that the two weapons perform best under different conditions.
Tailocins, the long-range weapon, only became effective when the attacking bacteria were at a high density and more common than the competition.
On the other hand, carrying toxin-loaded filaments gave a competitive advantage over a much greater range of conditions.
This included situations when the attacking bacteria were only present in low initial numbers and had to compete with a larger population of susceptible bacteria.
The researchers then challenged the two engineered strains in direct head-to-head competitions.

When the strains started at an equal frequency, the bacteria carrying toxin-loaded filaments had a distinct advantage.
However, both weapon users were able to win when they started in the majority.
Moreover, when cells could use both weapons simultaneously, they were able to suppress susceptible bacteria significantly better than strains that used only one weapon, demonstrating that the short- and long-range weapons complemented each other.
According to the researchers, the results show that short- and long-range weapons perform differently depending on the competition scenario.
Co-author Dr Sean Booth (University of Oxford) said: ‘Our results demonstrate that a particular advantage of contact-dependent weapons is that they are effective even when users are at a numerical disadvantage.
This suggests that they may have evolved to enable bacteria to invade an established population, when they are outnumbered by resident bacteria.’
This theory was supported by a computational model which simulated a low number of attacker cells attacking a larger population of susceptible cells.
In the model, cells using short-range weapons were able to successfully invade the community, whereas cells using long-range weapons were not.
However, when cells using long-range weapons were present in large numbers and were more common than the competition, these became extremely effective, giving the attackers a significant competitive advantage.
The researchers are now investigating how to apply the findings to custom-design beneficial microorganisms that can out-compete pathogenic strains.



