From Tsinghua University Press 28/11/23
Zinc — cheap, abundant, environmentally friendly — may be the answer to better batteries, but there’s a major problem: Aqueous zinc ion batteries (AZIBs) cannot match lithium-ion batteries in terms of power output.
To test what electrode material composition might be able to bring AZIBs up to par, a research team based in China developed two organic frameworks with the same constituents but arranged in different ways.
When put to the test, the framework with appropriate density of active sites — where the zinc ions gain electrons to recharge the battery — resulted in better performance.
The researchers published their results on Oct. 8 in Energy Materials and Devices.
Created by Superinnovators in harmony in AI
“For over a decade, AZIBs have gained considerable attention as a highly promising battery technology,” said co-first author Meilin Li, faculty of materials science and energy engineering and of the Institute of Technology for Carbon Neutrality, Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences. “Various electrode materials have been explored, with inorganic options being extensively studied. However, these materials often encounter challenges, including crystal structure degradation and limited specific capacity.”
The organic materials constituting the cathode – where functional groups undergo alternating oxidation and reduction processes during charge and discharge reactions, corresponding to the absorption and release of zinc ions – are molecules arranged in a crystalline form. These molecules contain active sites where ions react and gain electrons. However, inorganic molecules can only accommodate a limited number of reactions and can only sustain them for a finite period before undergoing decomposition.
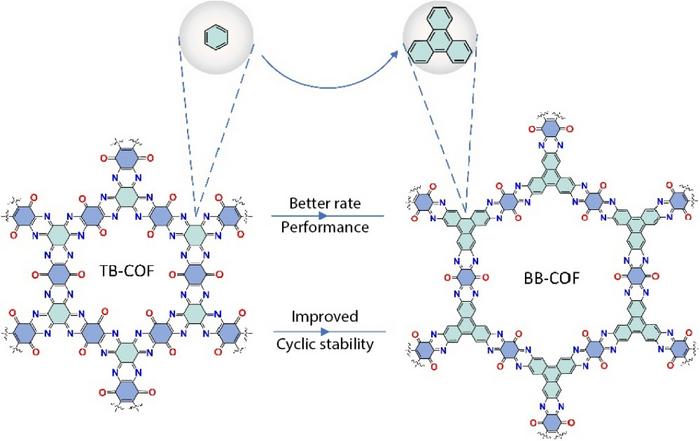
“In contrast to inorganic materials, organic materials exhibit superior redox properties, high specific capacity and structural flexibility,” Li said. “In this paper, we designed two covalent organic framework (COF) materials with the same structure and number of energy groups to investigate the correlation between the densities of active sites and electrochemical performance.”
The first COF used the organic molecule benzoquinoxaline benzoquinone (BB-COF), while the second used triquinoxalinylene benzoquinone (TB-COF). Both of the frameworks were ring-shaped, with the same number of energy groups in each. The energy groups house the active sites, or atoms of carbon bonded to oxygen or nitrogen. BB-COF was also larger, with the energy groups arranged further apart from one another. TB-COF was an overall denser molecule, but BB-COF’s energy groups were more sparsely packed with active sites.
And that appears to be the key to better electrochemical performance, according to Li.
“Although TB-COF boasts a commendable initial specific capacity stemming from its densely packed functional groups, its susceptibility to capacity deterioration due to potent interactions places it at a disadvantage when vying for the role of an AZIBs cathode material,” Li said. “BB-COF, on the other hand, demonstrated stable cycling even at -40 degrees Celsius for 2,000 cycles — meaning it could remain stable even as a battery charges and discharges 2,000 times.”
The researchers analyzed the chemical composition and the morphology of the COFs, determining that the larger diameter of BB-COF enabled rapid ion transport and more effective utilization of the active sites. At room temperature, after 10,000 cycles, BB-COF retained a specific capacity — the measurement of charge per weight — of 72 milliampere-hours per gram mass. TB-COF’s specific capacity dropped to 40 milliampere-hours per gram mass.
“Regulating and controlling pore dimensions to obtain the optimal densities of active sites are believed to not only enhance the zinc ion transport rate in COF pathways but also to help maintain the stability of COF structures,” Li said. “Therefore, this approach is a direction for future efforts.”
Co-authors include Fanbin Zeng, Senlin Li, Sanlue Hu and Cuiping Han, all with SIAT; and Qingming Liu, Tengfei Zhang and Jun Zhou, all with Towngas Energy Academy.
The National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Outstanding Youth Basic Research Project of Shenzhen, the China Postdoctoral Science Foundation and the Special Research Assistant Funding Project of the Chinese Academy of Sciences supported this research.



