From University of Cambridge 22/09/23
Imagine if humans could ‘talk’ to plants and warn them of approaching pest attacks or extreme weather.
A team of plant scientists at the Sainsbury Laboratory Cambridge University (SLCU) would like to turn this science fiction into reality using light-based messaging to ‘talk’ to plants.
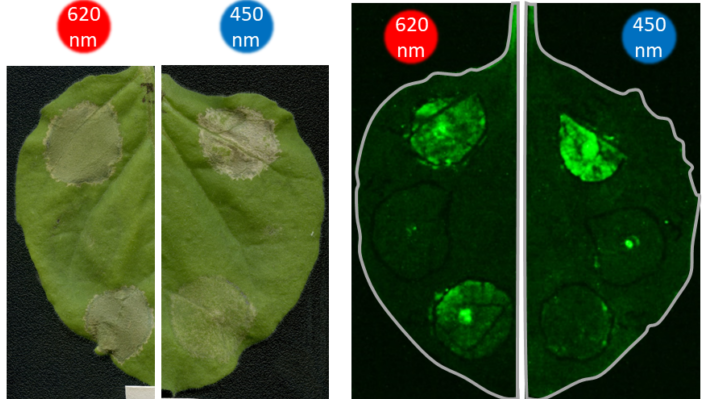
Early lab experiments with tobacco (Nicotiana benthamiana) have demonstrated that they can activate the plant’s natural defence mechanism (immune response) using light as a stimulus (messenger).
Light serves as a universal means of daily human communication, for example the signalling at traffic lights, pedestrian crossings, or the open-closed status of a shop.
Alexander Jones’ research team is using light as a messenger in the development of tools that enable plants to communicate with humans and humans to communicate with plants.
The University of Cambridge team previously engineered a series of biosensors using fluorescent light to visually communicate in real-time what is happening at the cellular level in plants, revealing the dynamics of critical plant hormones.
These biosensors can tell us how plants are reacting to environmental stresses – plants ‘talking’ to humans.
Their latest research published in PLOS Biology, describes a new tool called Highlighter, which uses specific light conditions to activate the expression of a target gene in plants, for example to trigger their defence mechanisms – humans ‘talking’ to plants.
The concept of humans being able to communicate with plants on a meaningful level has long captured the imagination of people.
If such a capability was possible, it could revolutionise agriculture and our relationship with plants.
“If we could warn plants of an impending disease outbreak or pest attack, plants could then activate their natural defence mechanisms to prevent widespread damage,” Dr Jones said.
“We could also inform plants about approaching extreme weather events, such as heatwaves or drought, allowing them to adjust their growth patterns or conserve water.
This could lead to more efficient and sustainable farming practices and reduce the need for chemicals.”
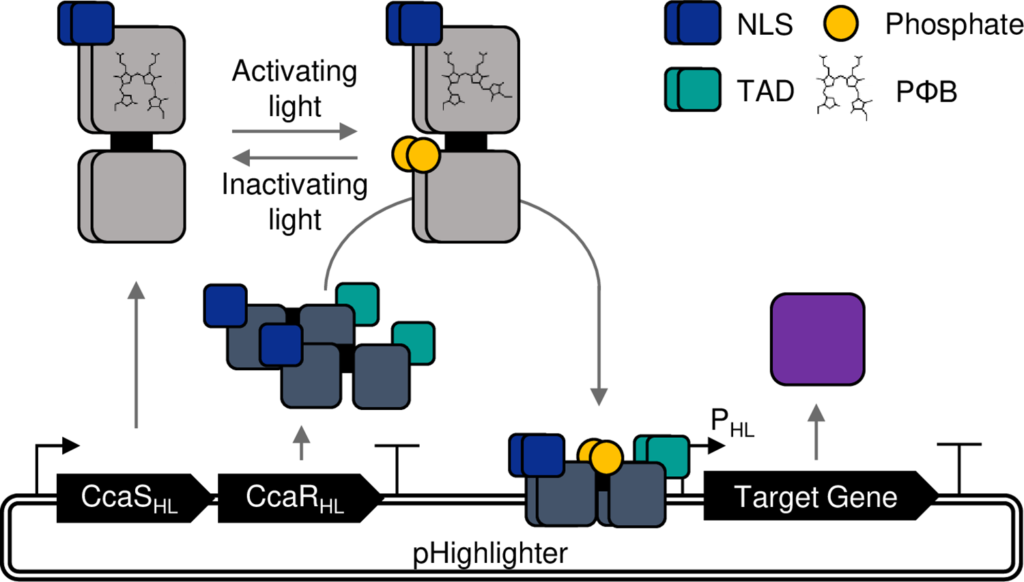
Bo Larsen, who engineered Highlighter while at SLCU, has taken us a major step closer to this goal of ‘talking’ to plants by engineering a light-controlled gene expression system (optogenetics system) from a prokaryotic system into a eukaryotic system that is tailored for plants.
Optogenetics can bring light to biomolecular processes in plants
To understand cellular activity biologists need to be able to control biomolecular processes at the cellular level. Optogenetics is a scientific technique that uses a light stimulus to activate or deactivate a specific process.
“Light stimuli are cheap, reversible, non-toxic and can be delivered with high-resolution,” Dr Jones said.
To do this, scientists engineer light-sensitive proteins (photoreceptors) to control a target process and then deliver these optogenetic ‘actuators’ to the cells they want to control.
Optogenetics has revolutionised many fields including neuroscience where biologists can isolate functions of individual neurons.
However, optogenetics has been difficult to apply to plants. This is because plants already contain lots of photoreceptors and need a wide spectrum of light to grow.
Switching from dark to light also activates native plant photoreceptors and a myriad of cellular systems.
Exacerbating this problem is the fact that many of the best performing optogenetic actuators use genetic parts from plants, meaning they could cross-talk with native photoreceptors if used in plants.

The story behind the research
Dr Jones, looking for an optogenetic gene expression switch that could be applied under normal horticultural light conditions without impacting on endogenous plant physiology and development, sought advice from J. Clark Lagarias, from UC Davis, who is an expert in phytochrome and cyanobacteriochrome light-switches.
He suggested repurposing the prokaryotic CcaS-CcaR optogenetic system, which was originally sourced from photosynthetic microbes and uses the ratio of green (on) – red (off) light signals.
By modulating the spectrum of white light plants need to grow, genes could be turned on or off using a minimally invasive stimulus.
But when developing Highlighter into a eukaryotic optogenetic system, Dr Larsen detected an unexpected blue-off behaviour.
Could the conversion have altered the green-red spectral properties of the CcaS photoreceptor?
Working together with Alex Jones, Ines Camacho and Richard Clarke from the National Physical Laboratory (NPL), they detected that the new system was still able to use green and red light just like the original system.
But the spectroscopic analysis at NPL also showed evidence of an independent blue-light sensing. Co-author Roberto Hofmann noticed that, in addition to the red-green sensing domain, CcaS had a domain with homology to blue-light photosensors called phototropins.
It seems the engineering efforts had inadvertently unlocked a latent CcaS blue sensing behaviour, providing an alternate way to control CcaS-CcaR activity.
Highlighter is an optogenetic tool for plants
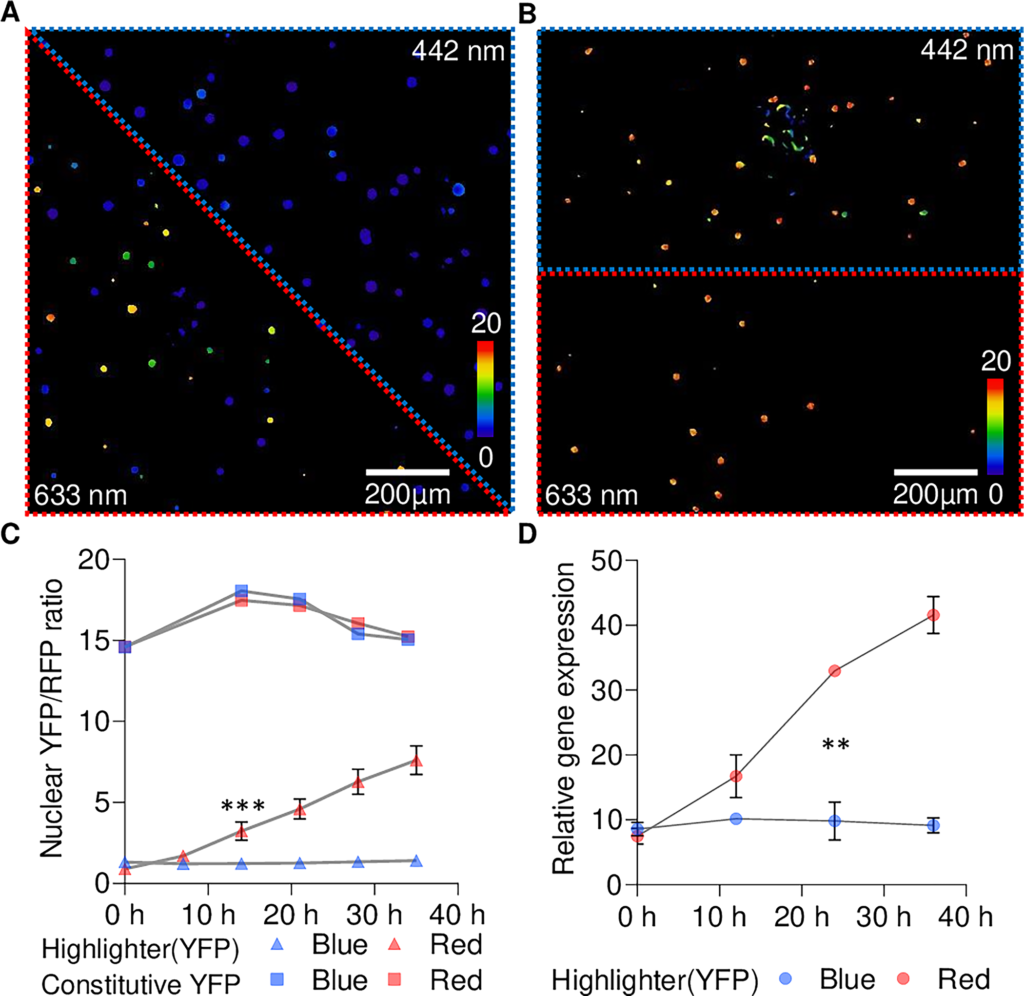
When deployed in plants, Highlighter uses minimally invasive light signals for activation and inactivation, and is unaffected by the light-dark cycling in growth chambers.
The current Highlighter system is inactive under blue light conditions and active in the dark and under white light, green light and, mysteriously, red light conditions.
Further work is planned to progress development of Highlighter, but the team has already demonstrated optogenetic control over plant immunity, pigment production and a yellow fluorescent protein, the latter at cellular resolution.
“Highlighter is an important step forward in the development of optogenetics tools in plants and its high-resolution gene control could be applied to study a large range of fundamental plant biology questions,” Dr Jones added.
“A growing toolbox for plants, with diverse optical properties, also opens exciting opportunities for crop improvement.
For example, in the future we could use one light condition to trigger an immune response, and then a different light condition to precisely time a particular trait, such as flowering or ripening.”